Hydrocyanic Acid Formula - Hydrocyanic Acid Uses, Properties, Structure and Formula
Hydrocyanic acid is the solution of hydrogen cyanide in water. It is a highly poisonous chemical, also called as prussic acid.
Formula and structure: The chemical formula of hydrocyanic acid is HCN. Its molecular formula is written as CHN and its molar mass is 27.03 g/mol. Hydrogen cyanide is a simple planar molecule, with a triple bond between the carbon and nitrogen atoms.
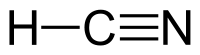
HCN has a structural isomer (tautomer) called hydrogen isocyanide (HNC).
Occurrence: HCN occurs naturally in the pits of certain fruits such as cherries, apples, and apricots. The fruit pits contain small amounts of cyanohydrins which form HCN.
Preparation: HCN is prepared on laboratory scale by the addition of acids to cyanide salts of alkali metals (such as NaCN, KCN, etc.):
HCl + NaCN → HCN + NaCl
The main industrial preparation is by the oxidation reaction of methane and ammonia at about 1200 °C, over a platinum catalyst (Andrussow oxidation):
2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
Physical properties: HCN is a pale blue or colorless transparent liquid (hydrocyanic acid) or a colorless gas (hydrogen cyanide). Hydrocyanic acid has a density of 0.687 g/mL, and boils slightly above room temperature, at 25.6 °C (78.1 °F). It has a distinct smell of bitter almonds, which is used to identify the presence of this highly poisonous material.
Chemical properties: Hydrogen cyanide is a weak acid, and partially ionizes in water to give H+ (or H3O+) and the cyanide anion, CN-.
HCN + H2O → H3O+ + CN-
It reacts with bases to form salts called cyanides.
HCN + KOH → KCN + H2O
Uses: Despite its toxicity, HCN is an important reagent used in the production of a variety of useful industrial chemicals such as sodium cyanide, potassium cyanide, methyl methacrylate (monomer used for making polymers and plastics), chelating agents EDTA and NTA, as well as the polymer Nylon. HCN is also used to prepare pesticides and chemical warfare agents.
Health hazards/ health effects: HCN is an extremely poisonous chemical, and is lethal in very small doses. A hydrogen cyanide concentration of a few hundred ppm in air can kill a human in less than one hour, and if ingested, it can cause almost immediate death. When high concentrations (of about 5.6%) of the HCN gas is exposed to air, it is also explosive.
|
Related Links: |
